Immunometabolic Diseases
Molecular Processes
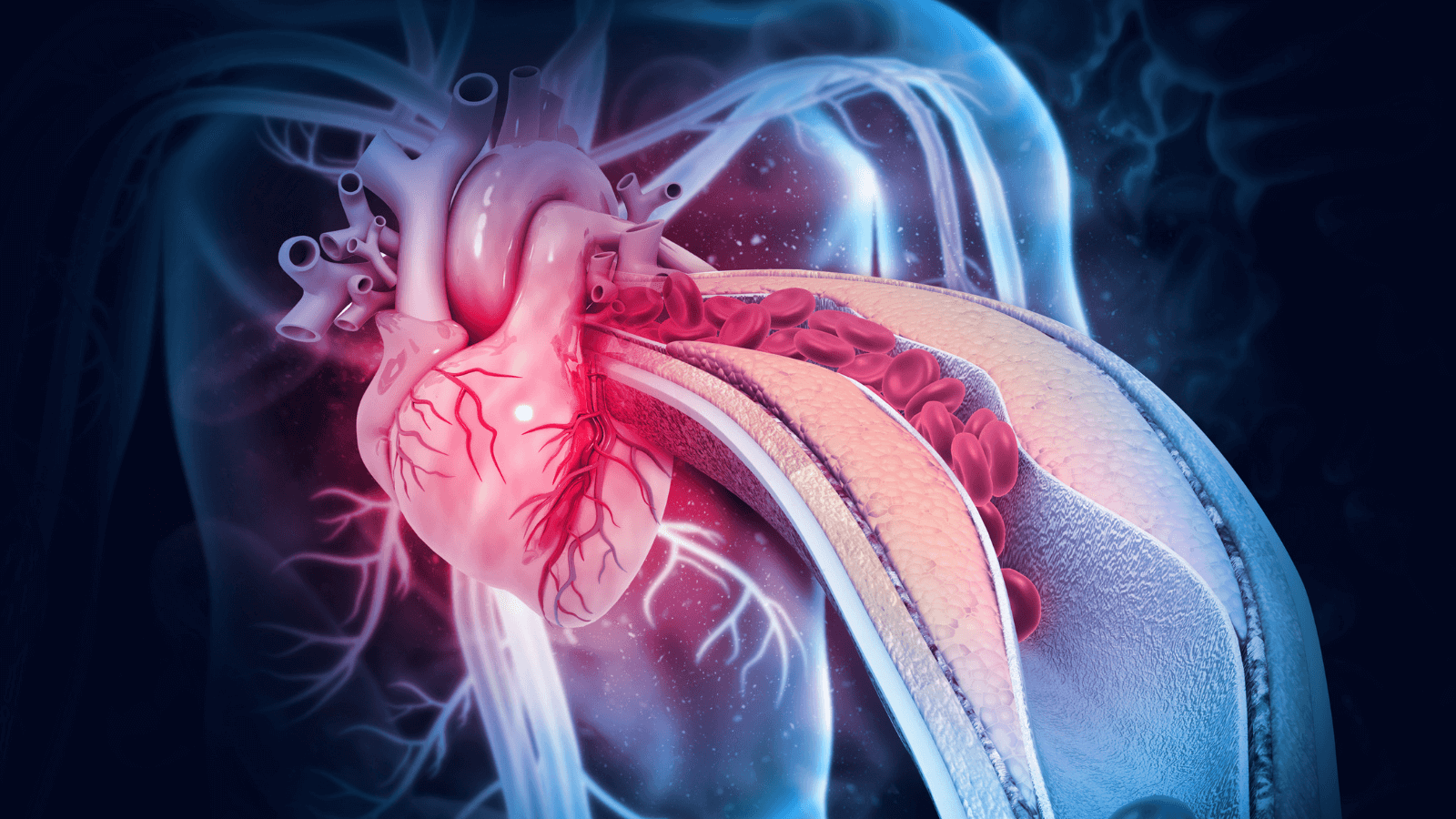
Atherosclerosis
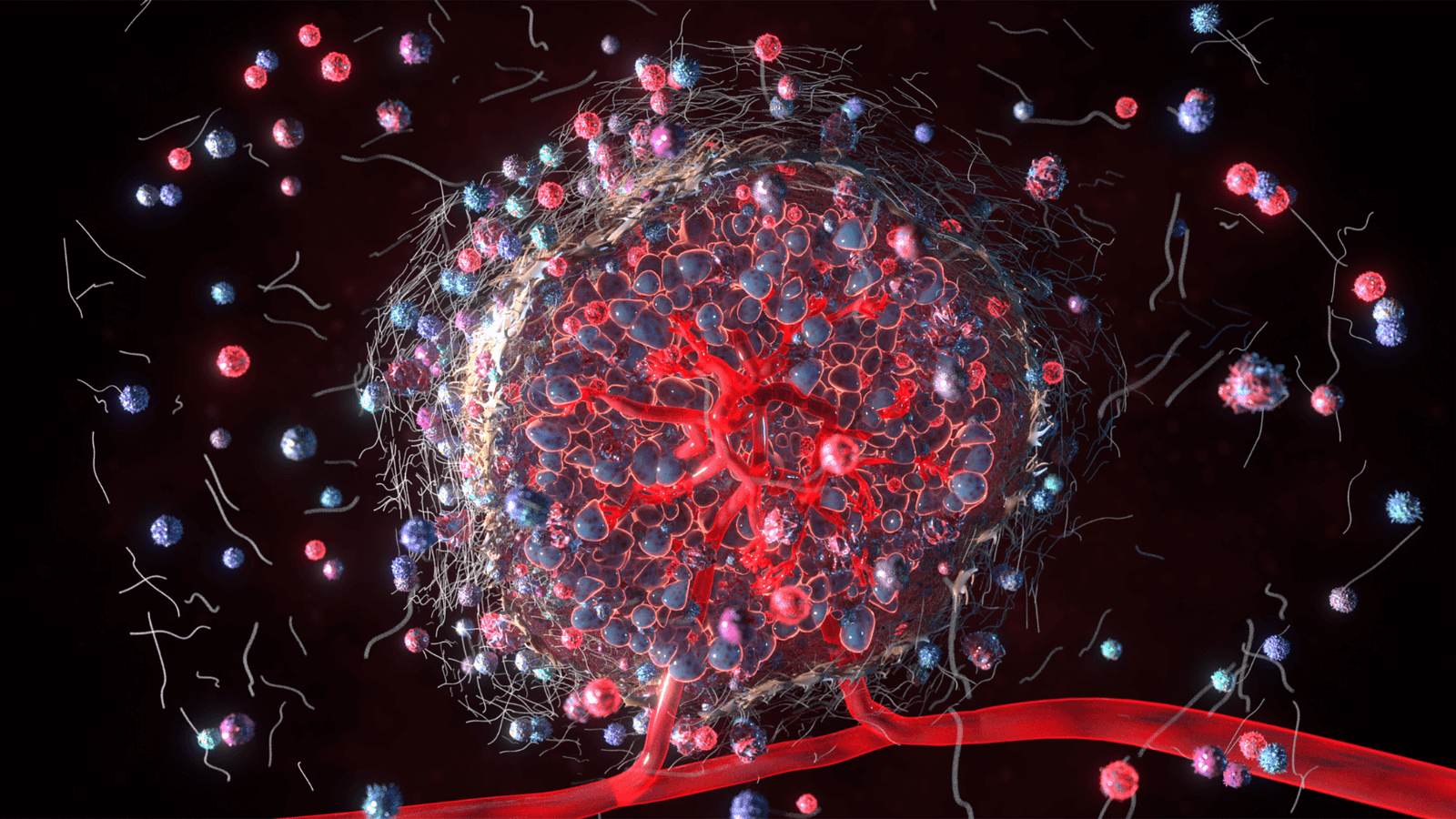
Macrophages are key players in the development and progression of atherosclerosis. Immunomodulatory roles of macrophages include engulfment and processing of excess lipids, including oxidized LDL and other oxidative moieties. Pathological alterations in autophagy and lipolysis contribute to plaque development, progression, and eventually instability.

Diabetes
Inflammation, immune system signaling, and immunometabolism are increasingly recognized as major contributors to the development of metabolic diseases, including Type 2 Diabetes. Tissue-specific immune responses in adipose, muscle, liver, pancreas, and the gastrointestinal system include activation of resident macrophages, infiltration of immune cells, inflammasome activation, oxidative stress, and immunomodulatory signaling. Immune system involvement in metabolism within these interconnected tissues and systemically is the physiological norm, and dysregulation and/or overburdening are consequently pathological.

Immune Cell Repertoire
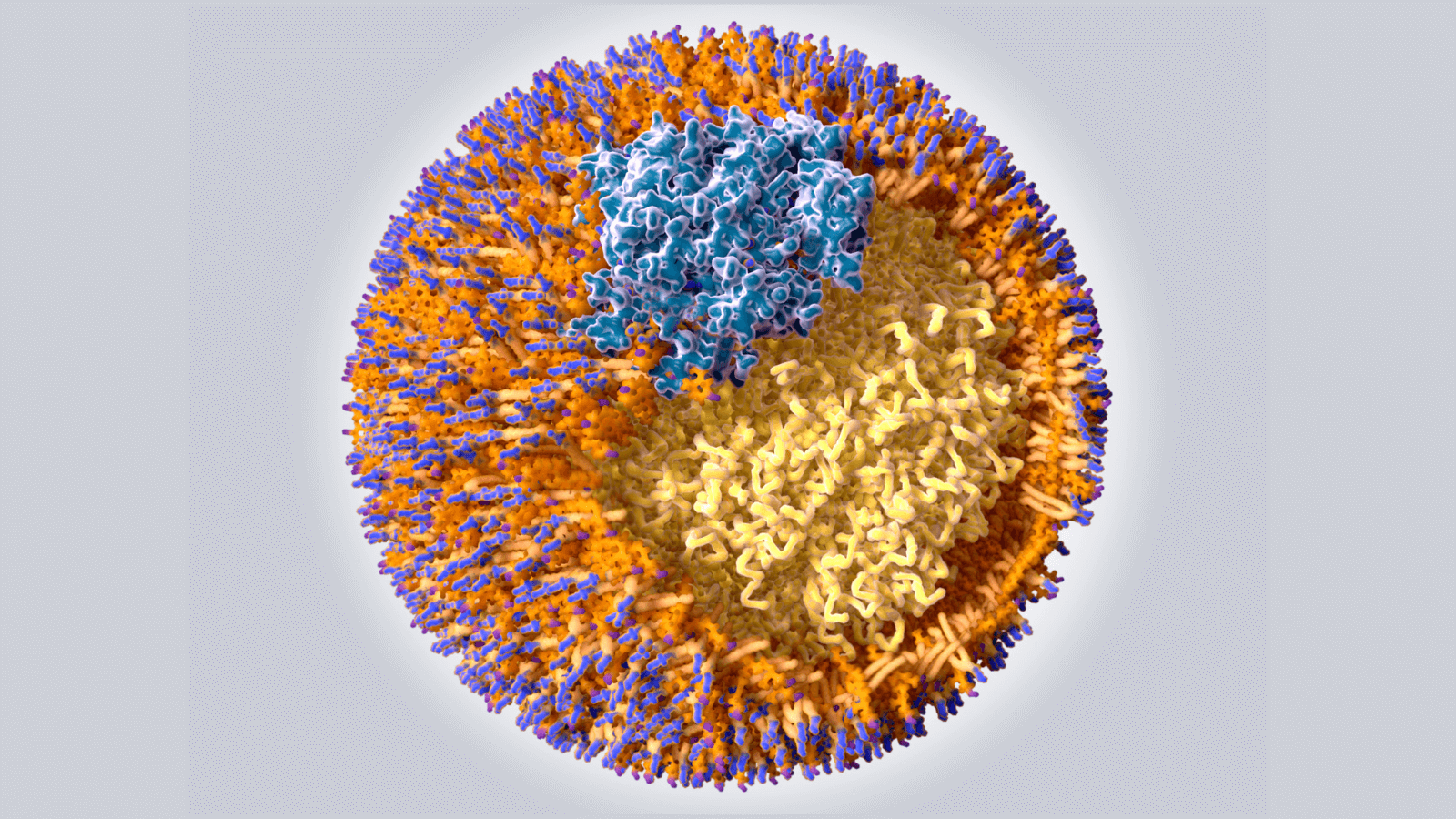
Lipid Metabolism
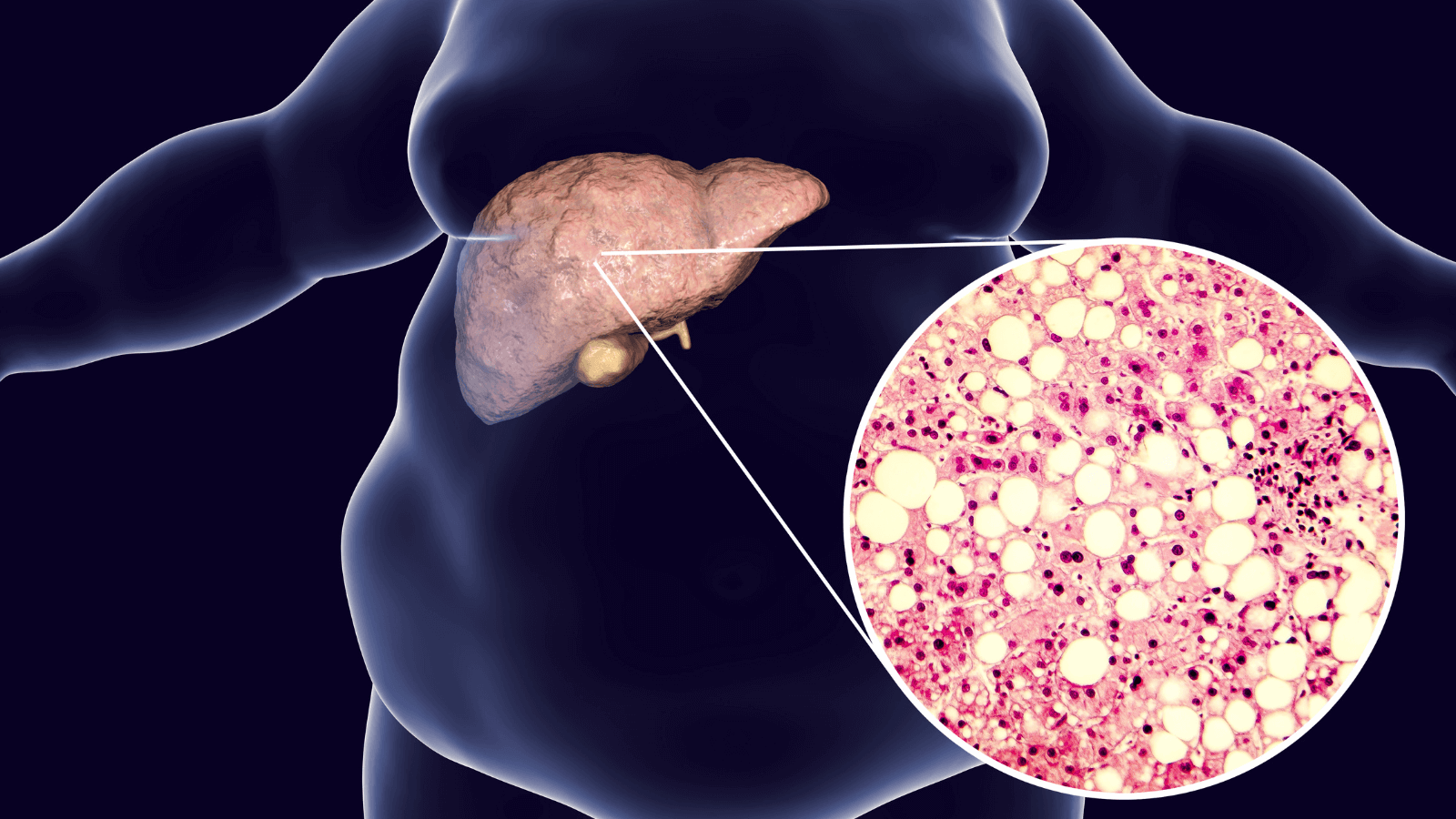
Metabolic Dysfunction-Associated Liver Disease
Metabolic inflammation in the liver contributes to the pathogenesis and progression of nonalcoholic liver disease. Liver macrophages are sub-specialized in processing lipids and helping to maintain metabolic homeostasis. Excess lipids, dysfunctional lipophagy, and inflammation are all major contributors to metabolic dysfunction-associated liver disease, which can lead to cirrhosis and liver failure.
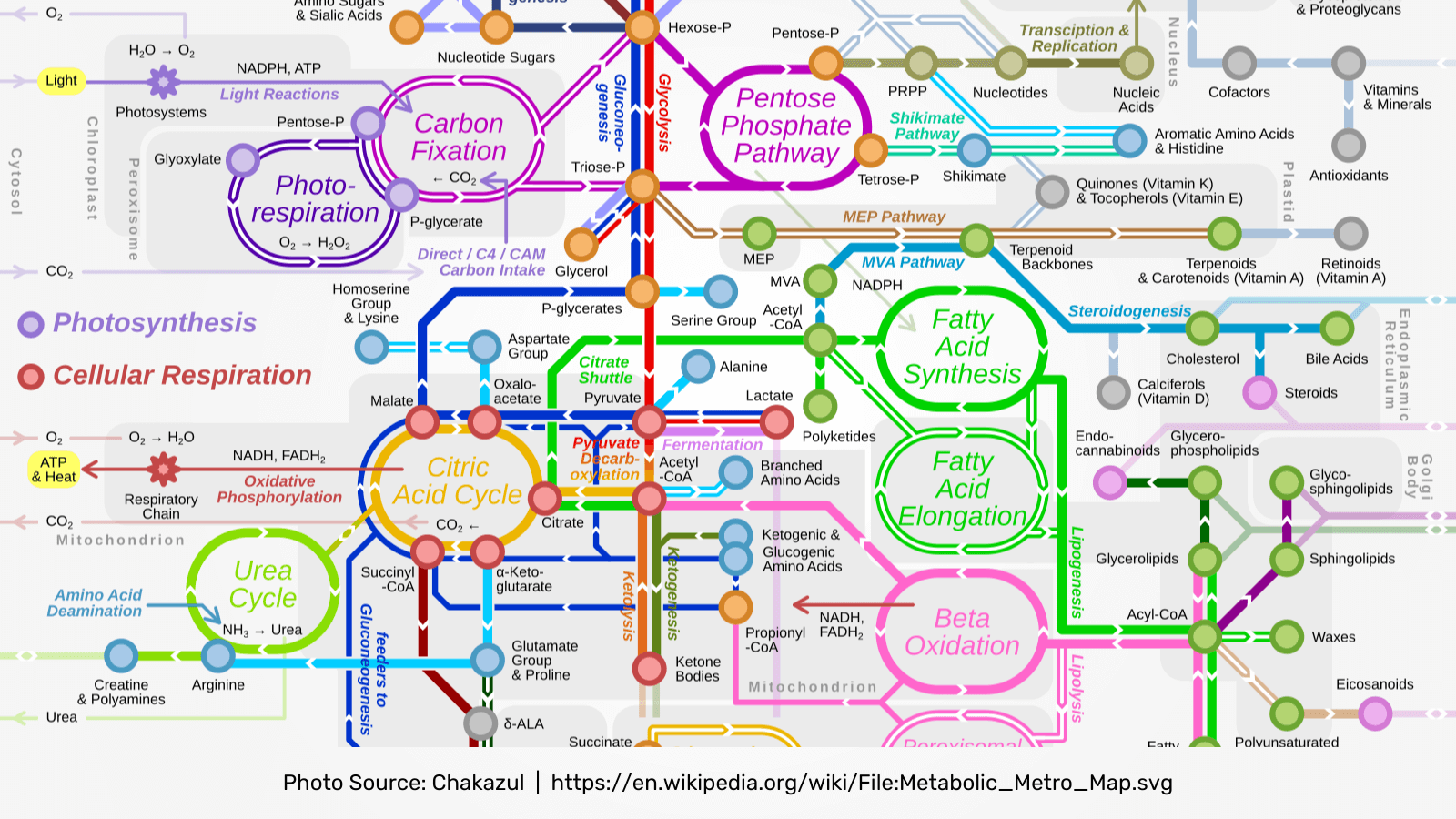
Metabolic Pathways
The most basic description of immunometabolism includes the changes that occur in intracellular metabolic pathways in immune cells during activation and as major metabolic mediators in resident tissues and organs. Major metabolic pathways in immune cells that are important under normal physiological and pathological conditions include glycolysis, the tricarboxylic acid (TCA) cycle, the pentose phosphate pathway, fatty acid oxidation, fatty acid synthesis and amino acid metabolism.

Mitochondria
Mitochondrial fitness, especially metabolism and energetics, regulate immune cell activation, and mitochondria are an essential component of immunometabolism within circulating and resident immune cells. Oxidative metabolism of glycolytic products, lipids, and amino acids are core processes governing mitochondrial energetics. Mitochondrial biosynthesis of intermediaries is also important for signaling, and mitochondrial sensing of cellular status adds to the overall dynamics of mitochondria-driven metabolic homeostasis.
Nutrient Sensing

Obesity
Adipose tissue serves as the body’s major energy repository, but recent studies have revealed that adipose tissue is not merely a lipid storage depot, but a dynamic and active metabolic organ involved in whole body metabolic homeostasis. Resident adipose tissue macrophages play crucial roles in maintaining normal metabolic functions and also contribute to hormonal coordination of multiple organ systems via adipokine signaling.
Pulmonary Disease